TOPStm System
Steven DeLuca, DO performed the first TOPS™ surgery in the U.S. at UPMC Pinnacle West Shore in Mechanicsburg in July 2017. The TOPS™ System is part of an FDA pivotal investigational device exemption (IDE) study sponsored by Philadelphia-based Premia Spine. The study evaluates the use of the TOPS™ System for patients with degenerative Grade I spondylolisthesis and spinal stenosis as an alternative to vertebrae fusion. The goal of the TOPS trial is to demonstrate patients can achieve greater range of motion in all directions with the investigational procedure.
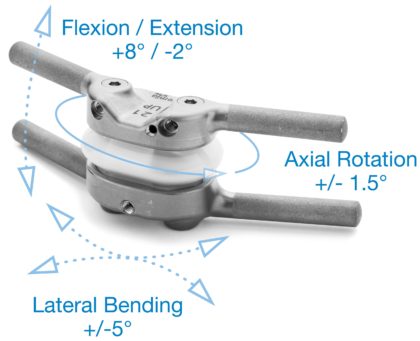
The TOPS™ System mechanical device is designed to restore motion of the spine in all directions. Instead of permanently locking the two vertebrae with a fusion, the device allows the two vertebrae to continue moving. UPMC Pinnacle is one of 30 spine centers throughout the U.S. taking part in the study to evaluate the efficacy and safety of the TOPS™ System compared to traditional lumbar fusion
Physicians who preform the TOPS procedure:
If interested please call:
(717) 761 -5530 x137